* sources Article publié dans Microscopy Today 00-1 pp. 8-12 (2000)
Formaldehyde, formalin, paraformaldehyde and glutaraldehyde:
what they are and what they do.
John A. Kiernan,
Department of Anatomy and Cell Biology,
University of Western Ontario, LONDON, Canada N6A 5C1
Aldehydes are the most commonly used fixatives. They are used to stabilize fine structural details of cells and tissues before examination by light or electron microscopy. Researchers, technicians, pathologists and others who regularly use aldehyde fixators often do not appreciate the nature and properties of these compounds or the reasons why they choose to fix a sample in formaldehyde, glutaraldehyde or a mixture both. Misconceptions are also common about formalin and paraformaldehyde, the commercial products from which solutions containing formaldehyde are made.
Properties of formaldehyde and its polymers
Formaldehyde is a gas. Its small molecules (HCHO, of which -CHO is the aldehyde group) dissolve quickly in water, with which they chemically combine to form methylene hydrate, HO-CH2-OH. It is the form in which formaldehyde exists in aqueous solutions; its chemical reactivity is the same as that of formaldehyde. The methylene hydrate molecules react with each other, combining to form polymers (Fig. 1). The liquid known as formaldehyde contains 37 to 40% formaldehyde and 60 to 63% water (by weight), most of the formaldehyde being in the form of low polymers (n = 2 to 8 in the formula given on the figure 1). The higher polymers (n up to 100), which are insoluble, are sold as a white powder, paraformaldehyde.
The hydrolysis of the polymers is catalyzed by the hydroxide ions present in the slightly alkaline solution (Fig. 1). Large molecules of paraformaldehyde polymer require more aggressive treatment. Heating is necessary, as is an additional source of hydroxide ions. In one of the first fixers derived from paraformaldehyde (Richardson, 1960), it was sodium sulfite, but the regular practice for at least 35 years has been simply to heat paraformaldehyde to 60 ° C in water containing the salts used to buffer the solution at pH 7.2. to 7.6.
Formalin contains about 10% methanol, added by the manufacturer because it slows down the polymerization which eventually leads to the precipitation of paraformaldehyde. A 4% formaldehyde solution based on formaldehyde therefore contains approximately 1% methanol. It also contains a small amount of formate ions. These are derived from the Cannizzaro reaction, in which two formaldehyde molecules react together, one being reduced to methanol and the other oxidized to formic acid. Due to this slow reaction, the concentrations of methanol and formate in any formaldehyde solution increase slowly with prolonged storage (Walker, 1964). A formaldehyde solution prepared from paraformaldehyde, which initially contains no methanol, is commonly used in fixatives for electron microscopy and in research applications. A satisfactory ultrastructural conservation is,
Reaction of formaldehyde with proteins
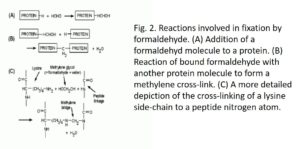
The aldehyde group can combine with nitrogen and certain other protein atoms, or with two of these atoms if they are very close to each other, forming a cross-linking -CH2- called methylene bridge. Tanning chemistry studies indicate that the most common type of crosslinking formed by formaldehyde in collagen is between the nitrogen atom at the end of the lysine side chain and the nitrogen atom of a peptide link (Fig. 2), and the number of these cross links increases over time (Gustavson, 1956). The tanning of collagen to make leather is comparable to the hardening of a fabric with a fixative (Hopwood, 1969). The fixative action of formaldehyde is probably entirely due to its reactions with proteins. The initial binding of formaldehyde to proteins is largely completed in 24 hours
(Helander, 1994) but the formation of methylene bridges takes place much more slowly. Substances such as carbohydrates, lipids and nucleic acids are trapped in a matrix of insolubilized and crosslinked protein molecules but are not chemically modified by formaldehyde, unless the fixation is prolonged for several weeks.
Practical considerations for formaldehyde
This is the most important element. Formaldehyde quickly enters tissues (small molecules), but its reactions with proteins, particularly cross-linking, occur slowly. Adequate fixation takes days, particularly if the sample has to withstand osmotic and other stresses of dehydration and infiltration with paraffin. A brief fixation in formaldehyde (ideally delivered by infusion) can significantly stop or reduce autolysis and provide mild hardening and some (but not much) resistance to fluids that are not iso-osmotic with the tissue. This can greatly improve the structural integrity of the cryostat and other frozen sections, especially if it is followed by infiltration with a cryoprotector such as sucrose (ideally 60% but more generally 15-30%).
When a sample is dehydrated after only a few hours in formaldehyde, the largely unbound cytoplasmic proteins are grossly coagulated. Nuclear chromatin, which contains DNA and strongly basic proteins, is also coagulated by the solvent, forming a pattern of threads, lumps and granules. This is no different from the appearance induced by fixatives which contain acetic acid, but it is less satisfactory for identifying cell types on the basis of nuclear morphology. (After adequate fixation with formaldehyde, the chromatin has a remarkably uniform texture, also of little diagnostic value but perhaps closer to the structure of the living nucleus.)
Glutaraldehyde solutions
Before 1962, the only satisfactory fixative for electron microscopy was buffered osmium tetroxide. This preserves cell structure by combining with lipids, especially in membranes, and by insolubilizing certain proteins without coagulation, but it is expensive and toxic, penetrates tissue extremely slowly and extracts a lot of protein and RNA. With the introduction of glutaraldehyde (Sabatini et al., 1962), electron microscopists had a faster penetrating fixative which completely insolubilized proteins and was inexpensive enough to be administered by vascular infusion.
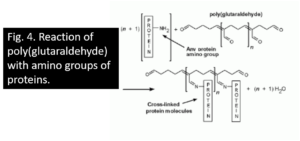
Glutaraldehyde has fairly small molecules, each with two aldehyde groups, separated by a flexible chain of 3 methylene bridges. It is HCO- (CH2) 3-CHO. The crosslinking potential is obviously much greater than with formaldehyde because it can occur both through the -CHO groups and over variable distances. In aqueous solutions, glutaraldehyde is mainly present in the form of polymers of variable size (Monsan et al., 1975). A free aldehyde group protrudes from the side of each unit of the polymer molecule (Figure 3), as well as one at each end. All of these -CHO groups will combine with all of the protein nitrogen they come into contact with, so there is enormous cross-linking potential, and that is exactly what is happening (Fig. 4). There are also many groups of remaining aldehydes (which are unrelated to anything) that cannot be washed out of the tissue.
Practical aspects of glutaraldehyde fixation
Five important points should be remembered when using glutaraldehyde as a fixative for light or electron microscopy.
1. If it is to be used as a fixative, especially for electron microscopy, the glutaraldehyde solution must contain the monomer and low polymers (oligomers) with molecules small enough to penetrate the tissue quickly enough. This means that you should purchase an “EM grade” glutaraldehyde (25% or 50% solution), not a
cheaper “technical” grade. The cheapest thing, which is for tanning leather, is largely made up of polymer molecules that are too large to fit between the macromolecules of cells and other tissue components.
2. The chemical reaction of glutaraldehyde with proteins is rapid (from a few minutes to a few hours), but the larger molecules, in particular the oligomers, penetrate slowly into the tissues. The brain of a rat left overnight in a buffered and sliced glutaraldehyde solution the next day shows a color change and a harder consistency up to a depth of 2-3 mm. Objects fixed for a few hours in glutaraldehyde no longer respond osmotically (Paljarvi et al., 1979).
3. The free aldehyde groups introduced by the fixing of glutaraldehyde pose various problems. These include non-specific binding of protein reagents, including antibodies, and a direct positive reaction with the Schiff reagent). Free aldehydes must be eliminated or blocked by appropriate histochemical procedures, as described in the manuals (Culling et al., 1985; Kiernan, 1999, Ruzin, 1999), before attempting immunohistochemistry, lectin histochemistry, Feulgen reaction of the periodic acid-Schiff coloration on material fixed with glutaraldehyde.
4. Thorough cross-linking of a glutaraldehyde-fixed sample prevents the penetration of large enough paraffin wax molecules. This makes cutting difficult and the differential differential removal artifacts inside the sample. You can well stain the mitochondria in cells surrounded by obviously abnormal spaces. This is an exaggeration of the insufficiency of formaldehyde and osmium tetroxide as fixatives to precede paraffin (Baker, 1958), and it also highlights the shortcomings of predominantly clotted fixators (AFA, Davidson, Bouin, etc.), which preserve micro-anatomy well but destroy or move small things like organelles. Fortunately, the plastic monomers correctly penetrate the tissues attached to glutaraldehyde. They have been shown not to fit into all crevices (Horobin & Tomlinson, 1976),
5. Immunohistochemistry, which requires as many side chains of intact amino acids as possible, is severely impaired by the binding of glutaraldehyde. Nevertheless, intelligent people have generated antibodies to individual amino acids, which are linked to glutaraldehyde and proteins. These allow the detection of neurotransmitters of soluble amino acids such as glutamate, GABA and even glycine in the presynaptic axonal terminals in the central nervous tissue perfused with glutaraldehyde (Hodgson et al., 1985; Hepler et al., 1988; Crooks & Kolb, 1992). Extensive crosslinking also results in the loss or severe reduction of most histochemically demonstrable enzymatic activities, although many are retained after brief fixation (Sabatini et al., 1962).
Mixtures containing formaldehyde and glutaraldehyde , this mixture is often designated Karnovsky fixative
The combination of formaldehyde with glutaraldehyde as a fixative for electron microscopy takes advantage of the rapid penetration of small HCHO molecules, which trigger the structural stabilization of the tissue. The rapid and complete crosslinking is caused by the slower penetrating glutaraldehyde oligomers. This mixture is associated with the name of Morris J. Karnovsky of Boston. This is an example of a great innovation that was only published in an unrevised summary (Karnovsky, 1965). Its original mixture contained 4% glutaraldehyde, which was a higher concentration than many people wanted to use (Hayat, 1981). Designations like
“Karnovsky at half strength” became common language in the 1960s and 1970s. Fixers of this type allowed for definitive descriptions of EM level histology that were accomplished in the next 5 or 6 years the introduction of Karnovsky’s fixer, and they are still commonly used.
The references
Baker, JR (1958). Principles of biological microtechnology (reprinted in 1970, with corrections). London: Methuen.
Carson, FL, Martin, JH & Lynn, JA (1973). Fixing formalin for